
Mars' atmosphere may be partially trapped within its surface. (© Artsiom P - stock.adobe.com)
In a nutshell
- NASA’s Curiosity rover discovered large deposits of siderite, a type of iron carbonate, in Gale Crater, offering the strongest in-situ evidence yet of an ancient carbon cycle on Mars.
- These carbon-rich rocks likely formed when Mars had a thicker, CO₂-rich atmosphere and liquid water, supporting the idea that the planet was once habitable.
- Some of that carbon was later released back into the atmosphere, suggesting that Mars experienced a dynamic carbon cycle that eventually contributed to its climate collapse and loss of habitability.
CALGARY, Canada — Mars once breathed carbon in and out just like Earth does today. NASA’s Curiosity rover has uncovered evidence that the Red Planet had its own version of Earth’s carbon cycle, a crucial planetary process that helps regulate climate and support life. By finding significant deposits of an iron carbonate mineral called siderite in Martian rocks, scientists have finally solved part of the mystery of where Mars’s thick, ancient atmosphere disappeared to.
On Earth, carbon dioxide moves between the air, oceans, and rocks in a balanced system. This study, published in Science, shows that a similar process once happened on Mars, but it was more of a one-way street. More carbon got trapped in rocks than returned to the atmosphere, eventually helping turn Mars into the cold, barren world we see today.
While climbing the slopes of a mountain inside Gale Crater, the Curiosity Rover drilled into rocks that scientists had suspected might contain interesting minerals. When they analyzed these samples, they unexpectedly found siderite, an iron-rich carbonate mineral, making up between 5% and 10% of the rock samples.
For siderite to form, you need liquid water, carbon dioxide, and the right chemical conditions. These are all essential ingredients for a potentially habitable environment.
The Mars Science Laboratory mission had long aimed at reaching these sulfate-packed rock formations. The team analyzed data from three of Curiosity’s drill sites that had siderite within sulfate-rich layers of Mount Sharp in Gale Crater.
“The abundance of highly soluble salts in these rocks and similar deposits mapped over much of Mars has been used as evidence of the ‘great drying’ of Mars during its dramatic shift from a warm and wet early Mars to its current, cold and dry state,” says lead author Ben Tutolo from the University of Calgary, in a statement.
The researchers believe these minerals formed in shallow subsurface environments where water was becoming increasingly limited. As the planet gradually dried up, more and more carbon dioxide from the atmosphere ended up trapped in these rocks.
The scientists also found evidence that some of this trapped carbon dioxide eventually made its way back into the atmosphere. Alongside the siderite, they discovered iron oxyhydroxides, minerals that form when siderite breaks down through later chemical reactions. This breakdown would have released some carbon dioxide back to the atmosphere, completing part of the carbon cycle.
With this in mind, the researchers estimated that similar rocks across Mars could have trapped the equivalent of up to 36 millibars of atmospheric carbon dioxide. For context, Mars’s current atmosphere contains only about 6 millibars of carbon dioxide, while Earth’s atmosphere, by comparison, has about 400 millibars of carbon dioxide.
“Sedimentary carbonate has long been predicted to have formed under the CO2-rich ancient Martian atmosphere, but identifications had previously been sparse,” says Tutolo.

University of Calgary 2025 Riley Brandt/University of Calgary)
The discovery of these carbonates suggests that the atmosphere contained enough carbon dioxide to support liquid water existing on the planet’s surface. As the atmosphere thinned, the carbon dioxide transformed into rock form.
This also explains why these carbonates haven’t been detected from orbit despite their abundance. They’re mixed with other minerals in a way that masks their spectral signature, making them invisible to orbital instruments. NASA suggests future missions and analysis of other sulfate-rich areas on Mars could confirm the findings and help to better understand the planet’s early history and how it transformed as its atmosphere was lost.
“The broader implications are the planet was habitable up until this time, but then, as the CO2 that had been warming the planet started to precipitate as siderite, it likely impacted Mars’ ability to stay warm,” adds Tutolo. “The question looking forward is how much of this CO2 from the atmosphere was actually sequestered? Was that potentially a reason we began to lose habitability?”
Tutolo also notes that this research connects to his ongoing work on Earth, where he is trying to turn anthropogenic CO2 into carbonates as a climate change solution. He emphasizes that Mars’ transition from a warm, wet planet to a cold, dry one highlights just how fragile a planet’s habitability is.
A planet we once viewed as simply dead and static emerges as a world that once had dynamic, Earth-like systems that ultimately failed to maintain habitability. This tells us not just about our neighbor planet, but about the delicate balance that has kept our own world livable for so long.
“The most remarkable thing about Earth is that it’s habitable and it has been for at least four billion years. Something happened to Mars that didn’t happen to Earth,” says Tutolo.
Paper Summary
Methodology
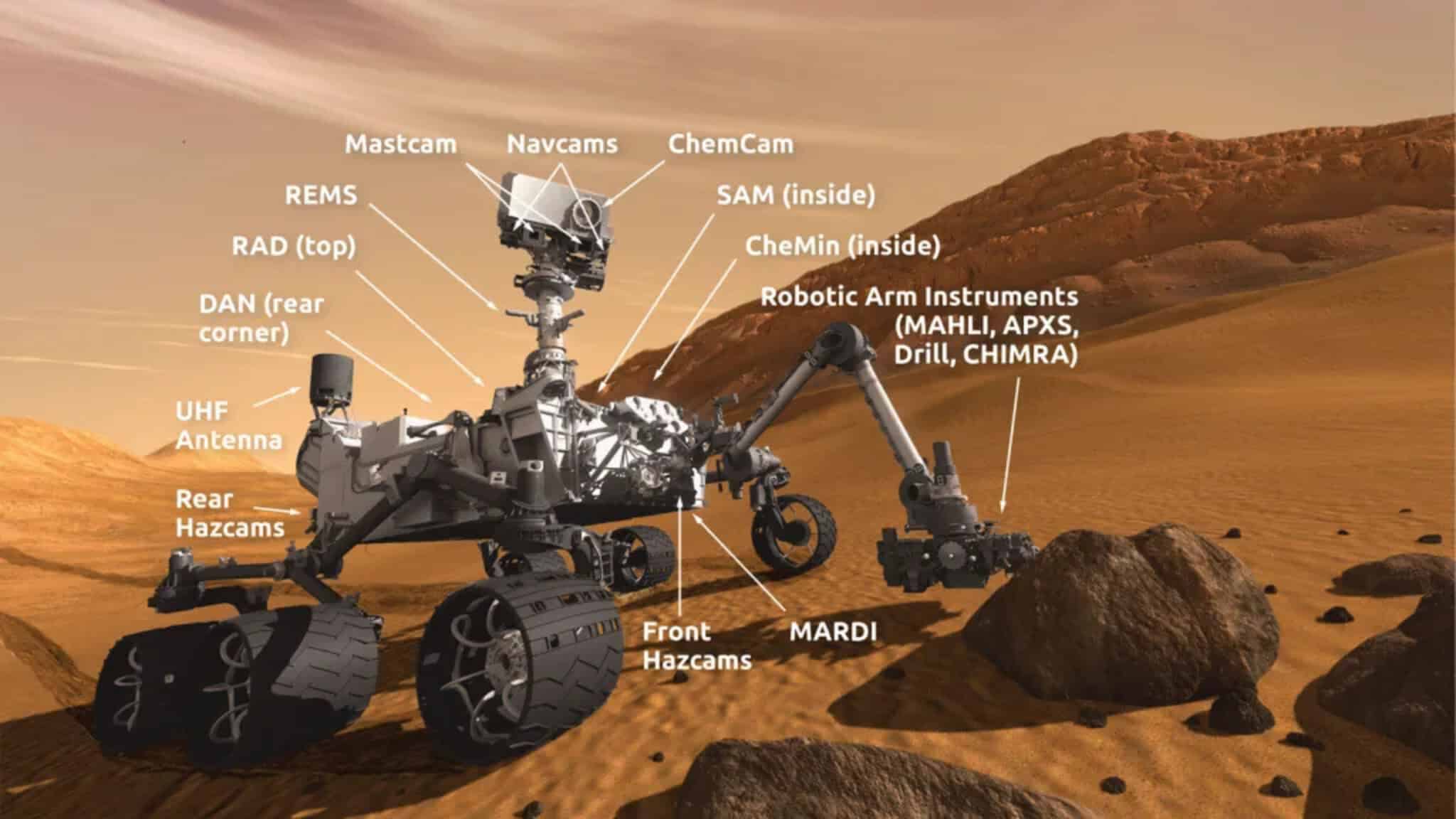
NASA’s Curiosity rover drilled four rock samples from different heights along an 89-meter section in Gale Crater, Mars. The samples—nicknamed Canaima, Tapo Caparo, Ubajara, and Sequoia—were analyzed using the rover’s built-in laboratory instruments. The main tool was CheMin (Chemistry and Mineralogy), which works like a high-tech X-ray machine to identify minerals in the samples. Another instrument called SAM (Sample Analysis at Mars) heated the samples and analyzed the gases released to confirm the findings. The rover also used its laser tool (ChemCam) to check the chemistry of rocks more frequently along its path.
Results
Three of the four samples contained significant amounts of siderite (iron carbonate)—between 5% and 10.5% by weight. Unlike other Martian carbonates found previously, these were almost pure iron carbonate with very little magnesium or calcium. The samples also contained basalt minerals, various sulfates, and iron oxide compounds. The researchers determined that the siderite formed when carbon dioxide-rich water interacted with iron in Martian rocks, followed by evaporation. The presence of iron oxides alongside the carbonates showed that some of the trapped carbon was later released back to the atmosphere through chemical reactions. Based on these findings, the team estimated that similar rocks across Mars might have trapped enough carbon dioxide to equal between 2.6 and 36 millibars of atmospheric pressure—potentially up to six times the amount in Mars’s current atmosphere.
Limitations
The study can’t precisely determine how much carbon dioxide was actually trapped across Mars because erosion has reduced the total volume of these deposits over time. The researchers also can’t say for certain whether the amount of CO2 in the ancient atmosphere would have been enough by itself to keep Mars warm enough for liquid water, as other factors might have contributed to warming. Additionally, they can’t pinpoint exactly when these minerals formed or how long the carbon cycle remained active on Mars.
Funding and Disclosures
The research received funding from multiple sources including the Canadian Space Agency, NASA, the UK Science and Technology Funding Council, and the UK Space Agency. Various team members were supported through different grants, including NASA’s Mars Exploration Program. Some of the research was conducted at NASA facilities including the Jet Propulsion Laboratory and NASA research centers. The authors declare that they have no competing interests.
Publication Information
The study, “Carbonates identified by the Curiosity rover indicate a carbon cycle operated on ancient Mars,” was published in Science (Volume 388, pages 292-297) on April 18, 2025. Benjamin M. Tutolo from the University of Calgary led the research, with over 30 co-authors from various institutions worldwide contributing to the work.







